
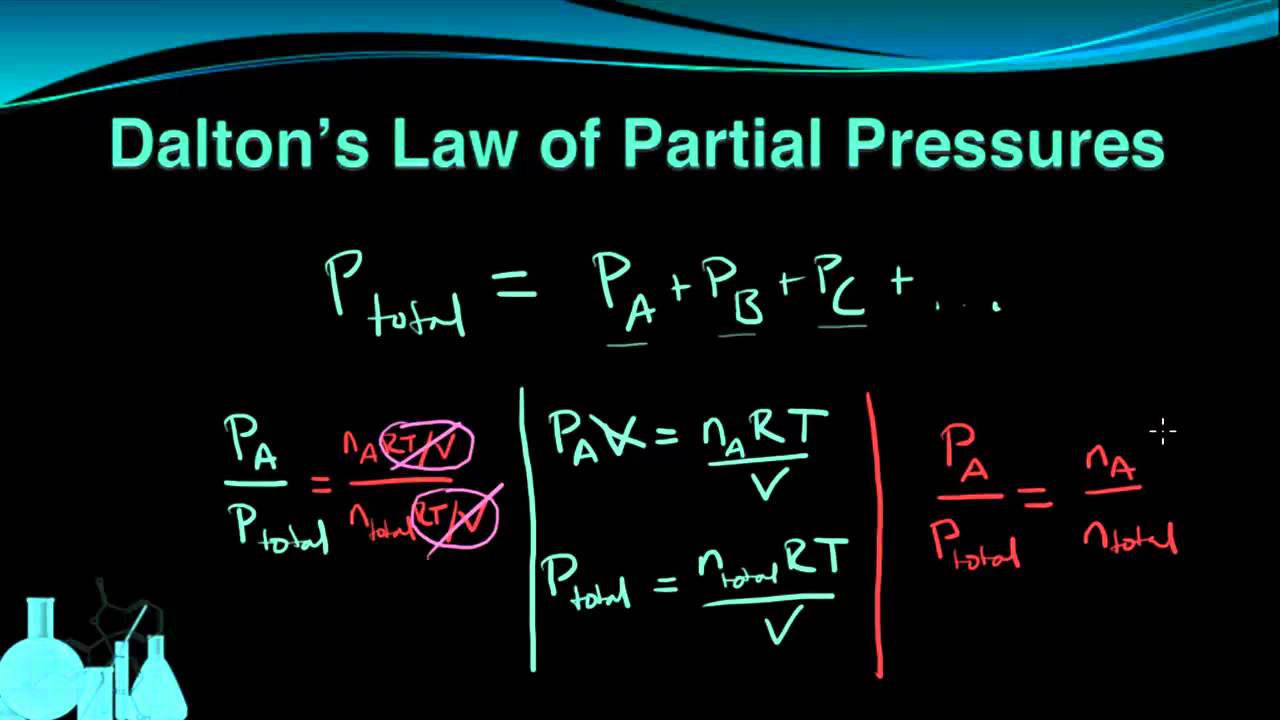
Let’s calculate the partial pressure using an example.
Pressure chemistry calculator how to#
You can find partial pressure easily by using partial pressure of gas calculator, however, you should also know how to calculate partial pressure of gas manually. Let’s go through partial pressure (Dalton’s law) definition, how to find partial pressure without using total pressure calculator, partial pressure equation, and some examples of Dalton’s law of partial pressure.ĭalton's law of partial pressure measures total pressure using temperature, moles of gas and volume.ĭalton’s Law states the principle that the pressure exerted by a mixture of gases in a fixed volume is equal to the sum of the pressures that each gas would exert if it occupied the whole volume. Pressure calculator is widely used in chemistry for pressure of gas calculation. It takes moles of gases, volume, and temperature from user to find the partial pressure of gases in the mixture. The Dalton’s law calculator finds the movement of gas in a solution. R is ideal gas constant with a value of 8.314 J K- 1 mol- 1. N i is the mount of moles of the individual gas, and P i is the partial pressure of the individual gas, Mole fraction is number of moles of individual gas divided by total number of moles in gas, P 1, p 2, p 3., p m is the partial pressures of the individual gases in the mixture, References:įrom the source of Wikipedia: Kinetic theory of gases, Equilibrium properties, Transport properties.įrom the source of Khan Academy: real gases, Deviations from ideal behavior, The van der Waals equation, Non-ideal behavior of gases.įrom the source of Lumen Learning: Charles’ and Gay-Lussac’s Law, Extrapolation to Zero Volume.Partial pressure = total pressure × mole fraction This is because this calculator gives most accurate results that are very crucial to avoid any disturbance in the reaction.
Pressure chemistry calculator free#
Chemists widely use free online partial pressure calculator while performing various chemical reactions. Without partial pressure, we are actually unable to make an estimation about gases.
This is because when water molecules freezes, they occupy 9% more space than before. Yes, it is true that ice always occupies more space than water. “The pressure that is exerted by a gas is always inversely proportional to the volume of the gas, keeping the temperature constant”. What does Boyle’s law demonstrate?īoyle’s law determines the relationship between a gas volume and pressure. The free henry’s law calculator determines:Ī hypothetical gas that consists of many point particles having no forces among them is called an ideal gas. In case you do choose Henry’s method 2, choose what next you wish to work for which can be either of the following: If you choose Henry’s method 1, make a further selection about what you wish to find from the following: If you select Ideal Gas Law, further make a choice about what you need to determine from the following in “To Calculate” drop down list: If you select Dalton’s law method, further select either of the given options in “To Calculate” drop down menu: For instance, let us guide you how to use it?įirst, you need to select one of the following methods: We have designed it in such a way that you will never feel difficulty in using it. But it does not mean that you can not use it. Well, our free calculator finds the accurate outputs regarding various important terms for gases. $$ Pressure = 0.384atm $$ How Partial Pressure Calculator Works? How to find partial pressure for which the solubility of the gas becomes \(2.4 * 10^-4\)M? We are given the value of Henry’s constant as \(2.3 * 10^3 L. “In a mixture of gases that is enclosed in a container, the total pressure exerted on the walls of the container is actually equal to the sum of the individual partial pressures exerted by each gas separately”ĭalton’s Law Of Partial Pressure Formula: Some of the important gas laws that are widely used to calculate the partial pressure are as follows: Dalton’s Law Of Partial Pressure: Uptill now many theories have been published for calculating partial pressure in a gas mixture. Partial pressures of some important gases are as follows: “In a mixture of gases, the pressure exerted by each individual gas separately is known as the partial pressure.” Keep reading! What Is A Partial Pressure? Before you move further, you need to understand important gas laws which are explained below. An online partial pressure calculator is properly designed to calculate partial pressure, volume, temperature, and amount of moles of each individual gas enclosed in a container.


 0 kommentar(er)
0 kommentar(er)
